Regeneron and Scholar Rock’s submissions have been delayed or denied following an FDA inspection at the third-party fill-finish facility Catalent Indiana, owned by Novo Nordisk.
A Canaccord analyst on Monday highlighted the risks associated with the U.S. Food and Drug Administration’s ‘Official Action Indicated’ designation on Catalent Indiana LLC, a third-party fill-finish facility.
Novo Nordisk’s (NVO) update that the FDA has classified Catalent Indiana LLC as “Official Action Indicated” raises the risk of further delay for Regeneron’s (REGN) Eylea HD prefilled syringes, as they are produced at the same site, the analyst noted. Catalent Indiana was acquired by Novo Nordisk (NVO) in December.
Notably, Regeneron had said in August that the FDA extended the date by which it is expected to rule on two of its EYLEA HD Injection regulatory submissions until the fourth quarter, owing to issues identified at the fill-finish facility.
Regeneron is not the only company whose submissions have been delayed or denied following the FDA’s site inspection at Catalent’s Indiana facility. Last month, the FDA refused to approve Scholar Rock’s (SRRK) Apitegromab drug for the treatment of patients with spinal muscular atrophy over concerns identified during the inspection of Catalent Indiana’s facility.
Breaches at Catalent Indiana’s facility have been classified as “Official Action Indicated,” Scholar Rock said in a statement. The serious designation means regulatory and/or administrative actions are recommended to resolve the concerns identified.
Scholar Rock said that it continues to work closely with Novo and has requested a meeting with the FDA to discuss the next steps for resubmitting its Apitegromab drug application.
While REGN shares traded 2% lower at the time of writing, SRRK stock fell 12%. While retail sentiment around REGN stock fell from ‘extremely bullish’ to ‘bullish’ territory over the past 24 hours, sentiment around SRRK rose from ‘neutral’ to ‘bullish’ territory.

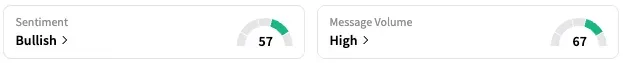
SRRK stock has dropped about 20% this year, while REGN lost 22%.
Read also: AbbVie Stock Rises After FDA Approves Changed Recommendations For Use Of Popular Rinvoq Drug
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
