MannKind’s stock has gained momentum amid optimism over its expanding diabetes portfolio and 2026 milestones.
- MannKind’s stock has gained momentum amid optimism over its expanding diabetes portfolio and 2026 milestones.
- CEO Michael Castagna said the FDA has accepted its Afrezza pediatric application, potentially making it the first new insulin for U.S. children in a century.
- MannKind is reviving its BlueHale smart inhaler and expanding in the $5.6 billion pediatric diabetes management market, according to Grand View Research.
MannKind shares are closing in on a breakout after a record quarter, with investors betting on its Afrezza pediatric approval — the first new insulin for U.S. children in a century — and the return of its connected inhaler platform, BlueHale.
Shares Rebound Toward Breakout Zone
Shares of MannKind have surged nearly 80% from their August low of $3.42, closing Wednesday at $6.06, within 6% of where they began the year, as investors focus on Afrezza’s next phase of growth and a series of 2026 milestones. The stock hit its highest levels in over 9 months on Wednesday and logged its best session in more than 2 months.
The company reported record third-quarter (Q3) revenue of $82 million, led by a 23% year-over-year rise in Afrezza inhaled insulin sales. Adjusted earnings of $0.07 per share beat consensus estimates of $0.02, according to Fiscal AI data.
Afrezza’s Pediatric Push
MannKind said the FDA has accepted its supplemental application to expand Afrezza to children aged 4 to 17, with a decision expected by May 2026. On the company’s Q3 earnings call, CEO Michael Castagna described the filing as a potential milestone that could make Afrezza the first new insulin for U.S. pediatric patients in more than a century.
Insulin therapy dates to 1922, when a 14-year-old boy became the first patient successfully treated, transforming type 1 diabetes from a fatal illness into a manageable condition, according to a Nature Medicine study archived by the U.S. National Library of Medicine.
The global pediatric diabetes management market, which includes devices, monitoring systems, and therapeutics for children and adolescents with diabetes, was valued at about $5.59 billion in 2024 and is projected to reach $13.11 billion by 2033, according to Grand View Research.
To prepare for the pediatric opportunity, MannKind is expanding its field and medical liaison teams, focusing on top pediatric diabetes centers, including Joslin Diabetes Center in Boston and the Barbara Davis Center in Denver.
The company also plans to roll out an updated Afrezza dose-conversion label early next year. Castagna said the change will help new prescribers correctly dose patients from the start and address “lack of effect,” which is the most common early complaint.
BlueHale Smart Inhaler Revived
MannKind is also reviving its BlueHale smart inhaler, which is a connected device that tracks insulin doses and integrates with continuous glucose monitors (CGMs). The latest version will feature reminders, dosing analytics, and time-in-range tracking to help patients and caregivers manage insulin use. The company plans to test BlueHale in its upcoming Inhaled First pediatric study, which aims to establish Afrezza as a first-choice bolus insulin for newly diagnosed youth with Type 1 diabetes.
Cardiometabolic Expansion And Pipeline Progress
In addition to diabetes, MannKind said the acquisition of scPharmaceuticals has strengthened its cardiometabolic portfolio.
A major milestone this quarter was the submission of the sNDA for the Furoscix ReadyFlow auto-injector. Upon approval, the Furoscix ReadyFlow auto-injector will simplify admissions, increase treatment options, and lower the cost of goods, creating capital that can be reinvested for growth and margin improvement.
The company is also advancing bumetanid DPI (MNKD-701) into preclinical development. Castagna said the inhaled formulation could serve patients who prefer inhaling rather than injecting. MannKind’s Technosphere platform has previously demonstrated IV-like onset and sustained efficacy in programs for insulin, treprostinil, and migraine, which the company believes supports the viability of MNKD-701.
Afrezza Upgrade Spurs Optimism On Stocktwits
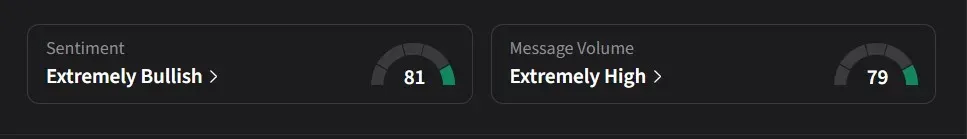
On Stocktwits, retail sentiment for MannKind was ‘extremely bullish’ amid a 360% surge in 24-hour message volume.
One user highlighted that MannKind’s upcoming Afrezza label update and the introduction of 2-unit cartridges could make dosing easier, improve “time in range,” and ultimately demonstrate Afrezza’s superiority over traditional insulins in future studies.
Another user described MannKind as a “deliberate, step-by-step growth story,” adding that they plan to increase their position in the stock next week.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
