FDA’s Fast Track designation is designed to facilitate the development and expedite the review of drugs intended to treat serious conditions and fill an unmet medical need.
Shares of Alto Neuroscience (ANRO) soared 54% on Friday afternoon after the company announced that the U.S. Food and Drug Administration granted Fast Track designation to ALTO-101 for the treatment of cognitive impairment associated with schizophrenia.
Cognitive impairment is a core and disabling feature of Schizophrenia, a serious, disabling mental illness affecting thought, perception, and behavior. According to Alto, there are currently no approved treatments for cognitive impairment associated with schizophrenia.
FDA’s Fast Track designation is designed to facilitate the development and expedite the review of drugs intended to treat serious conditions and fill an unmet medical need. A company, whose drug has a Fast Track designation, may be eligible for more frequent meetings with the FDA to discuss the drug’s development plan, as well as for accelerated approval and priority review, subject to the fulfillment of relevant criteria.
ALTO-101 is an inhibitor of the PDE4 enzyme that plays a key role in the brain by breaking down a molecule fundamental to learning and memory. By inhibiting the enzyme, the drug seeks to increase the levels of the molecule believed to improve cognitive function.
Alto CEO Amit Etkin termed the designation a significant milestone. “Our Phase 1 data, which demonstrated significant and clinically relevant effects of ALTO-101 on both EEG measures and cognitive performance in healthy subjects, provides strong validation for its mechanism,” he added.
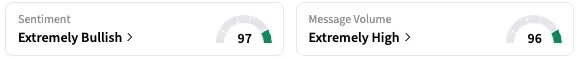
On Stocktwits, retail sentiment around ANRO stock rose from ‘bearish’ to ‘extremely bullish’ territory over the past 24 hours, while message volume rose from ‘low’ to ‘extremely high’ levels.
A Stocktwits user highlighted the company’s strong pipeline. Alto is seeking to develop treatments for mental health conditions, including bipolar depression, major depressive disorder, treatment-resistant depression (TRD), and schizophrenia.
Another user believes the FDA’s Fast Track designation is the icing on the cake.
ANRO stock is up by 61% this year but down by 33% over the past 12 months.
Read also: Rivian Reportedly Eyes Door Redesign To Alleviate Safety Concerns: Report
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
