Six-month data from 40 participants suggest that Revita helps limit weight regain after GLP-1 discontinuation.
- The results reinforce the device’s potential durability and support continued study in the pivotal REMAIN-1 trial.
- Beyond weight maintenance, Revita-treated participants demonstrated improvements in lipid profiles.
- Patient-reported outcomes also indicated reduced cravings for sweet foods.
Fractyl Health, Inc. (GUTS) announced on Thursday positive six-month findings from its ongoing REMAIN-1 Midpoint Cohort, a study evaluating Revita for weight maintenance after patients discontinue GLP-1 therapy.
The six-month data, covering 40 participants, suggest that Revita helps limit weight regain following GLP-1 discontinuation, with treated patients regaining 4.5% of weight versus 7.5% in the sham group.
Midpoint Study Shows Promising Results
An exploratory analysis of those who lost above-median weight during the GLP-1 run-in showed a 70% relative reduction in post-GLP-1 weight regain for Revita-treated participants compared with sham.
These results reinforce the device’s potential durability and support continued study in the pivotal REMAIN-1 trial. Beyond weight maintenance, Revita-treated participants demonstrated improvements in lipid profiles, including higher HDL cholesterol and a lower triglyceride-to-HDL ratio.
Patient-reported outcomes also indicated reduced cravings for sweet foods, suggesting that the device may influence appetite and metabolism through gut-mediated mechanisms.
However, Fractyl Health’s stock plummeted 63% in Thursday’s premarket.
How Did Stocktwits Users React
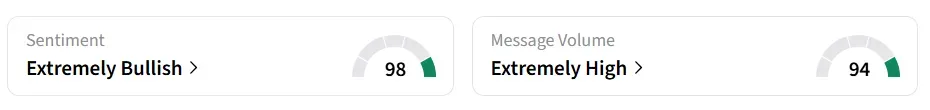
Despite the fall in the stock’s price, retail sentiment around the stock remained in ‘extremely bullish’ territory amid ‘extremely high’ message volume levels.
A bullish Stocktwits user said the product that reduces sweet cravings could be extremely profitable, highlighting huge market potential.
Another user said they trusted the management and would hold the stock.
Revita, the main product from Fractyl Health, is meant to reshape the lining of the small intestine through a single, minimally invasive procedure. The FDA has granted Revita Breakthrough Device designation to help people maintain weight after stopping GLP-1 treatments.
The company also outlined upcoming catalysts, including one‑year cohort readouts and pivotal REMAIN‑1 data later this year, which could support a future U.S. marketing application.
GUTS stock has declined by over 9% in the last 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
