In the ROSELLA trial, patients receiving relacorilant with nab-paclitaxel saw their risk of death drop by about 35% compared with those given the chemotherapy alone.
- Median overall survival was 16 months for the combination versus 11.9 months for the group that received nab-paclitaxel alone.
- Earlier data from the same trial showed a roughly 30% reduction in the risk of disease progression for participants treated with relacorilant plus nab-paclitaxel.
- ROSELLA enrolled 381 women with platinum-resistant ovarian cancer across multiple regions.
Corcept Therapeutics Inc. (CORT) on Thursday announced positive clinical results from its Phase 3 ROSELLA study evaluating the experimental drug relacorilant combined with nab-paclitaxel chemotherapy in women with platinum-resistant ovarian cancer.
The company said the study hit its key survival goal, underscoring potential new hope for patients.
Positive Survival Results
In the ROSELLA trial, patients receiving relacorilant with nab-paclitaxel saw their risk of death drop by about 35% compared with those given the chemotherapy alone.
Median overall survival was 16.0 months for the combination versus 11.9 months for the group that received nab-paclitaxel alone, the company said.
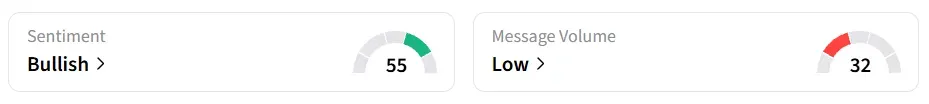
Following the results, Corcept stock traded over 14% higher on Thursday morning. On Stocktwits, retail sentiment around the stock jumped to ‘bullish’ from ‘bearish’ territory the previous day. Message volume changed to ‘low’ from ‘extremely low’ levels in 24 hours.
Progression-Free Survival
Earlier data from the same trial showed a roughly 30% reduction in the risk of disease progression for participants treated with relacorilant plus nab-paclitaxel. Safety findings for the combination therapy align with established profiles, with no new adverse events emerging, suggesting the drug can deliver a meaningful clinical benefit without adding extra risk.
“The addition of relacorilant to nab-paclitaxel, a trusted and effective chemotherapy, is positioned to become a new standard-of-care treatment for patients with platinum-resistant ovarian cancer, due to its overall survival benefit, well-tolerated side effect profile and oral administration.”
-Alexander B. Olawaiye, Principal Investigator, ROSELLA Trial
ROSELLA enrolled 381 women with platinum-resistant ovarian cancer across multiple regions, including the United States, Europe, South Korea, Brazil, Argentina, Canada, and Australia.
CORT stock has declined by 31% in the last 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.
