Rezolute said that its Phase 3 sunRIZE trial of ersodetug to treat low blood pressure failed to meet its primary endpoint.
- RZLT stock dropped to its lowest level since February 2024.
- The study also missed its key secondary endpoint, the company said.
- Rezolute said that its Phase 3 upLIFT study in tumour-related hyperinsulinism is underway, with topline results expected in the second half of 2026.
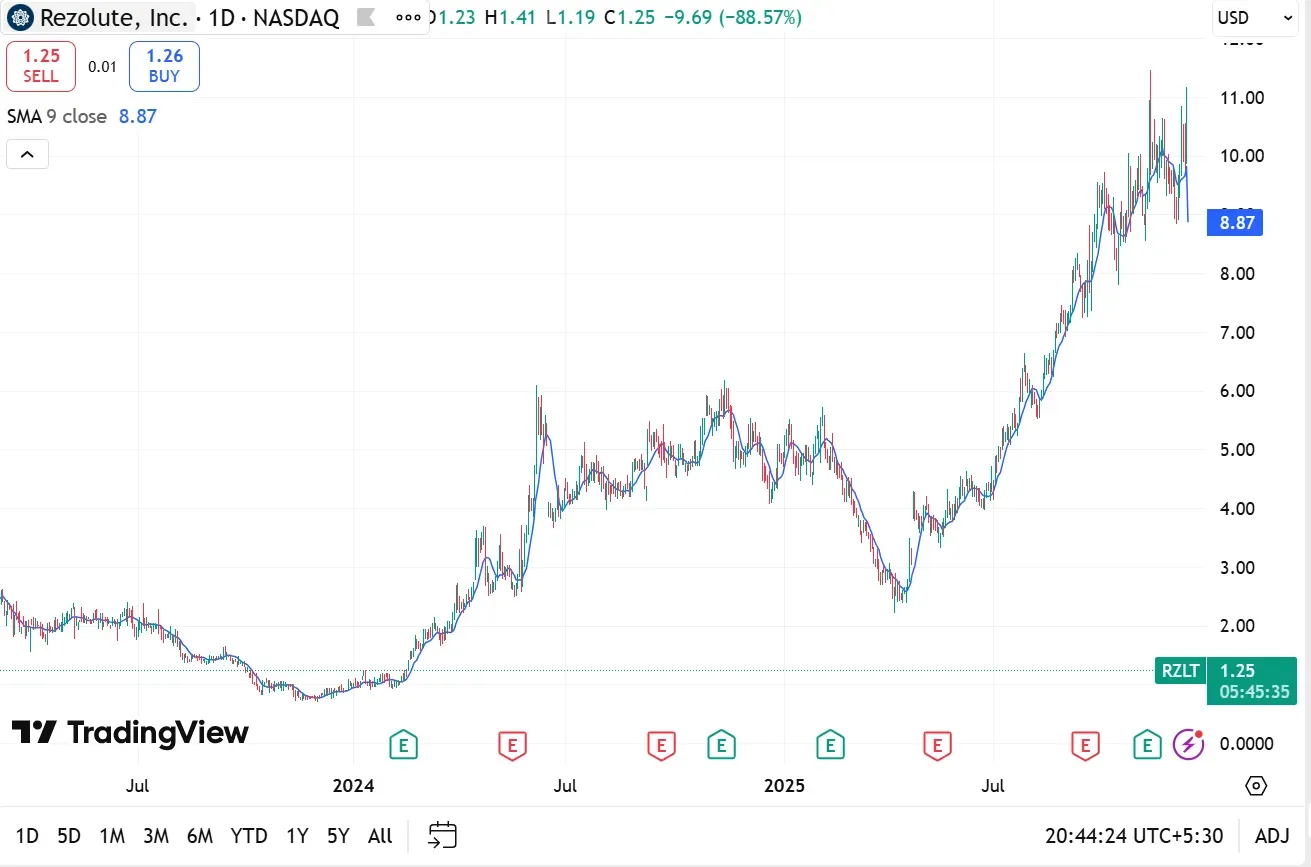
Shares of Rezolute, Inc. (RZLT) collapsed nearly 90% on Thursday, after the company reported that its Phase 3 sunRIZE trial of ersodetug to treat low blood pressure failed to meet its primary endpoint.
Following the decline, RZLT stock fell below its 200-day moving average (200-DMA) for the first time since April 2024 and dropped to its lowest level since February 2024, with trading halted just minutes after the opening bell.
What Did The Study Show?
Rezolute reported topline Phase 3 sunRIZE results showing that ersodetug failed to meet its primary endpoint in patients with congenital hyperinsulinism, as the treatment did not reduce weekly hypoglycemia events based on self-monitored blood glucose readings.
At the highest dose of 10 mg/kg, ersodetug showed a roughly 45% reduction in hypoglycemia events, but this was not statistically significant compared with the placebo group, which saw a 40% improvement.
The study also missed its key secondary endpoint, which assessed changes in daily time spent in hypoglycemia through continuous glucose monitoring. Patients on the 10 mg/kg dose experienced about a 25% reduction in time spent in low blood sugar, but this improvement did not differ significantly from placebo, which showed a 5% increase.
Despite the lack of efficacy signals, Rezolute said the achieved drug levels were consistent with expectations across all ages, and the overall safety profile was favorable. Two participants experienced severe hypersensitivity reactions that required stopping treatment, but the company noted that the overall incidence of serious allergic reactions was low. The most common side effect was hypertrichosis, a condition that causes temporary hair growth.
“We are disappointed that the study did not demonstrate significant improvements in glucose-related endpoints relative to placebo as well as for the patients and families living with congenital HI who urgently need new treatment options. At the same time, there are aspects of the results that merit additional investigation, and we are conducting a thorough evaluation to gain a better understanding of the study outcomes, which will inform our path forward,” said Brian Roberts, Chief Medical Officer of Rezolute.
“We intend to meet with FDA under our Breakthrough Therapy Designation to consider next steps for the program.”
Rezolute said that its Phase 3 upLIFT study in tumour-related hyperinsulinism is underway, and topline results are expected in the second half of 2026.
How Did Stocktwits Users React?
Despite the intraday decline, retail sentiment on Stocktwits remained ‘extremely bullish’ over the past 24 hours, accompanied by ‘extremely high’ message volumes.
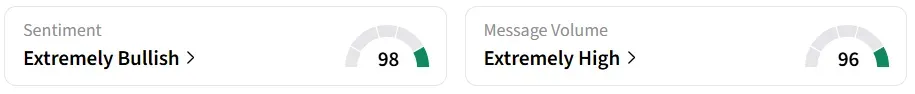
A Stocktwits user believes the stock is oversold.
The stock is down over 73% this year.
Read also: CRBP Stock Surged 36% Pre-Market – What Did Its Obesity Treatment Drug Study Reveal?
For updates and corrections, email newsroom[at]stocktwits[dot]com.<