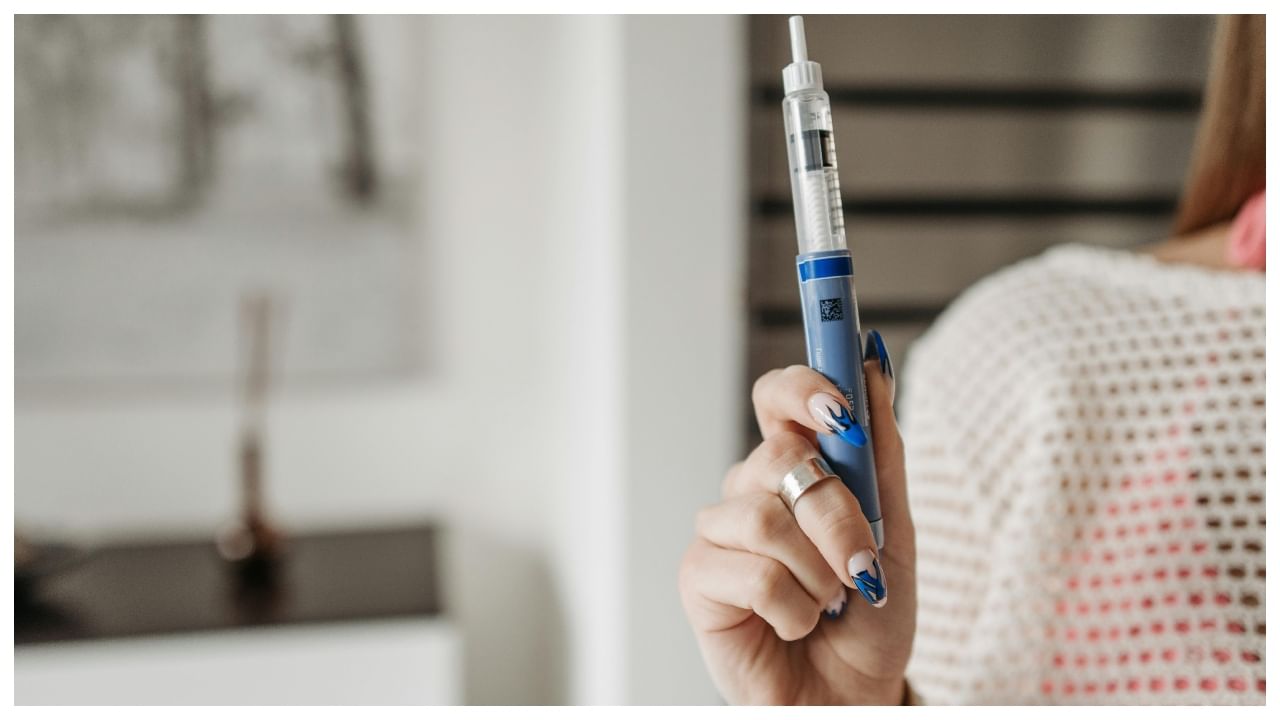
New Delhi: Novo Nordisk is preparing to introduce its blockbuster diabetes drug Ozempic to the Indian market, with the launch expected within the next few weeks, according to people familiar with the company’s plans, Reuters claimed. The Danish pharmaceutical giant has been steadily expanding its presence in India, and the arrival of Ozempic marks a significant move in a country grappling with fast-rising rates of type 2 diabetes and obesity.
India now has the world’s second-largest population of diabetes patients, trailing only China. With obesity also climbing sharply, the market for weight-management medication is expanding at a rapid pace. Analysts believe the global weight-loss drug sector could reach $150 billion a year by the end of the decade, and manufacturers are racing to secure an early advantage in key regions.
Ozempic, a once-weekly injectable approved in the United States in 2017, has become one of Novo Nordisk’s most successful products. Although it is officially cleared for diabetes management, its appetite-reducing properties have fuelled widespread off-label use for weight loss. Its sister drug, Wegovy, contains the same active ingredient — semaglutide — and is specifically approved for weight reduction.
Industry sources say Novo Nordisk is eager to introduce Ozempic in India before lower-cost generic competitors appear. The company received regulatory clearance to import and sell the drug earlier this year and has signalled that it hopes to bring it to market as soon as possible. Pricing, however, remains a sensitive issue. Vikrant Shrotriya, who heads Novo Nordisk India, said the firm is working to keep costs competitive given India’s price-conscious healthcare landscape.
The launch also comes at a time of fierce competition. Eli Lilly’s Mounjaro — approved for both diabetes and weight loss — has surged ahead in India, becoming the highest-selling drug by value in October. Sales of Mounjaro that month stood at 262,000 doses, dwarfing the 26,000 doses of Wegovy sold during the same period. Novo Nordisk recently slashed Wegovy’s price by up to 37 per cent, anticipating that its semaglutide patent will expire in 2026, opening the door to inexpensive generics.
Indian pharmaceutical companies are already working on their own semaglutide versions. Major players such as Sun Pharma, Cipla, Dr. Reddy’s and Lupin are developing alternatives to tap into the booming demand for weight-loss treatments.
Analysts say Novo Nordisk’s existing presence in India’s diabetes-care sector — particularly through its Rybelsus tablets — gives it a solid platform for Ozempic’s rollout. Some doctors may also prescribe the drug for related conditions such as sleep apnea, infertility linked to metabolic issues, or general weight management.
Ozempic, Wegovy and Mounjaro all fall under the GLP-1 class of drugs, created for diabetes but now widely used for their ability to slow digestion and increase feelings of fullness.