Following the announcement, H.C. Wainwright downgraded IO Biotech to ‘Neutral’ from ‘Buy’ with no price target.
IO Biotech (IOBT) stock slumped 77% by Monday afternoon after the company announced that the U.S. Food and Drug Administration (FDA) recommended that the firm conduct another trial before submitting Cylembio for approval.
IO Biotech held a meeting with the agency, which recommended that the firm not submit an application based on data from the IOB-013 clinical trial, in which Cylembio plus Merck’s Pembrolizumab improved progression-free survival (PFS), but narrowly missed statistical significance.
“We look forward to continuing the dialogue with FDA to align on the design for a potential new registrational study. Additionally, we plan to discuss the data from our IOB-013 study with European regulators and determine a path to submission in the EU,” CEO Mai-Britt Zocca said.
The company also stated that it is implementing a plan to conserve capital while seeking approval for the drug and conducting ongoing studies. IO Biotech currently has capital to sustain its operation into the first quarter of 2026. However, it is now planning restructuring, including a halving of its workforce.
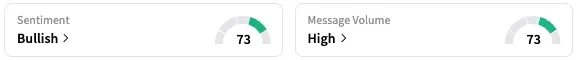
On Stocktwits, retail sentiment around IOBT stock jumped from ‘neutral’ to ‘extremely bullish’ territory over the past 24 hours, while message volume rose from ‘normal’ to ‘extremely high’ levels.
Following the company’s announcement, H.C. Wainwright downgraded IO Biotech to ‘Neutral’ from ‘Buy’ with no price target, as per TheFly. Given its cash position, the company would either need to raise additional capital, partner, or license out Cylembio to advance the new trial, the analyst noted.
IO Biotech had announced in August that the late-stage trial of its investigational and therapeutic cancer vaccine, Cylembio, in combination with Pembrolizumab, failed to achieve statistical significance in terms of progression-free survival improvement compared to Keytruda alone.
The trial evaluated Cylembio in combination with Keytruda versus Keytruda alone in 407 patients with previously untreated, unresectable, or metastatic (advanced) melanoma. Patients in the study treated with Cylembio in combination with Keytruda achieved a median progression-free survival of 19.4 months, compared to 11.0 months in patients treated with Keytruda alone. Progression-free survival (PFS) refers to the duration of time during and after cancer treatment during which a patient lives without the disease worsening.
IOBT stock is down 61% this year and approximately 66% over the past 12 months.
Read also: Enanta Pharmaceuticals Stock Rises 58% – Here’s An Important Update
For updates and corrections, email newsroom[at]stocktwits[dot]com.<