The company reported that a lower hospitalization rate was observed in patients treated with Zelicapavir compared to those treated with placebo, and the drug demonstrated a robust antiviral effect.
Enanta Pharmaceuticals (ENTA) on Monday announced that its mid-stage trial evaluating the efficacy and safety of Zelicapavir in outpatient adults with acute Respiratory Syncytial Virus (RSV) infection who are at high risk of complications failed to meet the primary endpoint.
Shares of the company, however, rose 58% by Monday afternoon. Notably, the company also stated that a clinically meaningful improvement in time to complete resolution of all 13 RSV symptoms was observed for Zelicapavir compared to placebo, with a benefit of 2.2 days for the overall efficacy population and 6.7 days for patients with congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), or aged 75 and older.
A lower hospitalization rate was observed for patients treated with Zelicapavir compared to placebo, and Zelicapavir demonstrated a robust antiviral effect, it added.
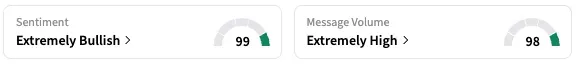
The study, however, failed in its primary endpoint of lowering the time to resolution of lower respiratory tract disease symptoms to mild. On Stocktwits, retail sentiment around ENTA stock stayed within the ‘extremely bullish’ territory over the past 24 hours, while message volume stayed at ‘extremely high’ levels.
A Stocktwits user cheered the rally.
Another noted that though the trial missed the primary endpoint, the rest of the data “looks great.”
Enanta said on Monday that Zelicapavir demonstrated a favorable safety profile in the trial and was well-tolerated. The goal of the study was to inform the design of a late-stage trial, including populations and endpoints, and no adverse events led to treatment discontinuation or study withdrawal in the Zelicapavir group.
“We believe the totality of these data provides strong rationale for further clinical advancement of zelicapavir. Importantly, we identified multiple potential registrational endpoints for a Phase 3 trial,” said Chief Medical Officer Scott T. Rottinghaus.
RSV is a common respiratory virus that infects the lungs and respiratory tract. Older adults are at significantly increased risk of severe illness due to the natural weakening of the immune system with age.
ENTA stock has more than doubled this year and gained by about 20% over the past 12 months.
Read also: Kala Bio Stock Just Plunged 89% Today – Here’s What Happened
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
